fluocinonide .05%
 Fluocinonide Cream 0.05% 60g
Fluocinonide Cream 0.05% 60gFLUOCINONIDE- Fluocinonide cream FLUOCINONIDE FLUOCINONIDE FLUOCINONIDE-fluocinonide unintment Taro Pharmaceuticals U.S.A., Inc. ---------- Fluocinonide USP of the cream, 0.05% USP of the Fluocinonide Cream, 0.05% (Emulsified Base) Fluocinonide Particle, 0.05% UTN of Fluocinonide ointment, 0.05% Only for external use. Not for ophthalmological use. Rx only DESCRIPTIONFluocinonide Cream USP, 0.05%, Fluocinonide Cream USP, 0.05% (Emulsified Base), Fluocinonide Gel USP, 0.05% and Fluocinonide Ointment USP, 0.05% are intended for topical administration. The active component in each is the corticosteroid fluocinonide, which is the 21-oil ster of the fluocinolone acetonide and has the pregna-1,4-dieno-3,20-dione,21-(acetiloxi)-6,9-difluoro-11-hydroxy-16,17-[1-metilintidene)bis(oxy) It has the following chemical structure: Fluocinonide Cream USP, 0.05% contains fluocinonide 0.5 mg/g in a specially formulated cream base consisting of citric acid, glycerin, 1,2,6-hexanetriol, gluco-3350 polyethylene, glucocol-8000 polyethylene, glucocol propylene and star alcohol. This white cream vehicle is ungrassable, contains no water, anhydrated and completely inert. The base provides emollient and hydrophilic properties. Fluocinonide Cream USP, 0.05% (Emulsified Base) contains fluocinonide 0.5 mg/g in a lavable emollient base of water of cetylic alcohol, citric acid (anhydrous), mineral oil, polysorbate 60, propylene glucocol, purified water, sorbitaneous monosteate, stellar alcohol and white gasoline. Fluocinonide Gel USP, 0.05% contains fluocinonide 0.5 mg/g in a specially formulated gel base consisting of carbomer 940, edetate disodium, propylene gallon, propylene glycol, sodium hydroxide (to adjust pH) and purified water. This light, colourless vehicle is without fat, without water and completely insolent. Fluocinonide Ointment USP, 0.05% contains fluocinonide 0.5 mg/g in a specially formulated ointment base consisting of glyceryl monosteate, propylene carbonate, propylene glycol, white gasoline and white wax. It provides the desired occluent and emollient effects in a ointment. In the USP of Fluocinonide Cream, 0.05%, Fluocinonide Gel USP, 0.05%, and Fluocinonide ointment UTP, formulations 0.05%, the active ingredient is totally in solution. CLINICAL PHARMACOLOGYOptic corticosteroids share anti-inflammatory, anti-pruritic and vasoconstrictive actions. The anti-inflammatory mechanism of topical corticosteroids is unclear. Various laboratory methods are used, including vasoconstrictor trials, to compare and predict potencies and/or clinical efficacys of topical corticosteroids. There are some tests that suggest that there is a recognizable correlation between vasoconstrictor power and therapeutic efficacy in man. Pharmacokinetics The scope of percutaneous absorption of topical corticosteroids is determined by many factors, including the vehicle, the integrity of the epidermal barrier, and the use of occlusive deposits. Topical corticosteroids can be absorbed from normal intact skin. Inflammation and/or other skin disease processes increase percutaneous absorption. Occlusive deposits substantially increase the percutaneous absorption of topical corticosteroids. Therefore, occlusive deposits can be a valuable therapeutic supplement for the treatment of resistant dermates (see ). Once absorbed by the skin, topical corticosteroids are handled through pharmacokinetic pathways similar to systemically administered corticosteroids. Corticosteroids are linked to plasma proteins in varying degrees. Corticosteroids are metabolized mainly in the liver and then excreted by the kidneys. Some of the topical corticosteroids and their metabolites are also excreted in the bile. INDICATIONS AND USAGEFluocinonide Cream USP, 0.05%, Fluocinonide Cream USP, 0.05% (Emulsified Base), Fluocinonide Gel USP, 0.05% and Fluocinonide Ointment USP, 0.05% is indicated for the relief of inflammatory and pruritic manifestations of corticoid-resistant dermates. CONTRAINDICATIONSOptic corticosteroids are contraindicated in patients with a history of hypersensitivity to any of the components of the preparations. PRECAUTIONSGeneralThe systemic absorption of topical corticosteroids has resulted in the suppression of the hypothalamic-adrenal axial axial (HPA), manifestations of Cushing syndrome, hyperglycemia and glucosuria in some patients. The conditions that increase systemic absorption include the application of the most powerful steroids, the use on large surfaces, the prolonged use and the addition of occlusive deposits. Therefore, patients who receive a large dose of a potent topical steroid applied to a large surface or under an occlusive deposit should be evaluated periodically for evidence of HPA axis removal using Urinary free cortisol tests and ACTH stimulation. If the removal of the HPA axis is observed, you should try to remove the medication, reduce the frequency of application or replace a less potent steroid. The recovery of the HPA axis function is usually quick and complete when the medication is left aside. Infrequently, signs and symptoms of steroid withdrawal may occur, which require additional systemic corticosteroids. Children can absorb proportionally larger amounts of topical corticosteroids and therefore be more susceptible to systemic toxicity (see ). If irritation develops, topical corticosteroids must be interrupted and appropriate therapy must be instituted. As with any topical corticosteroid product, prolonged use can produce atrophy of the skin and subcutaneous tissues. When used in intertwined or flexor areas, or in the face, this can occur even with short-term use. In the presence of dermatological infections, the use of an appropriate antifungal or antibacterial agent should be instituted. If a favorable response does not occur quickly, corticosteroids should be suspended until the infection has been properly controlled. Patient information Patients who use topical corticosteroids should receive the following information and instructions: Laboratory tests The following tests may be useful for evaluating removal of the HPA axis:Carcinogenesis, Mutagenesis and fertility impairment Long-term animal studies have not been conducted to evaluate the carcinogenic potential or the effect on the fertility of the topical corticoids. Studies to determine mutagenicity with prednisolone and hydrocortisone have revealed negative results. Pregnancy Category Corticosteroids are usually teatrogens in laboratory animals when administered systemically at relatively low doses levels. The most potent corticosteroids have proven to be teratogens after dermal application in laboratory animals. There are no adequate and well-controlled studies in pregnant women on topical corticosteroid teatrogen effects. Therefore, topical corticosteroids should be used during pregnancy only if the potential benefit justifies the potential risk for the fetus. This type of medication should not be used extensively in pregnant patients, in large quantities or for long periods of time. Mothers of Nursing It is not known whether the topical administration of corticosteroids could lead to enough systemic absorption to produce detectable amounts in breast milk. Systematically administered corticosteroids are secreted in breast milk in quantities that are not likely to have a harmful effect on the baby. However, precaution should be exercised when topical corticosteroids are given to a woman in nursing. Pediatric Use Pediatric patients can demonstrate greater susceptibility to the suppression of the hypotalmic-adrenal axis (HPA) induced by corticosteroids and Cushing syndrome than mature patients due to a greater surface of the skin to the body weight ratio. The removal of the HPA axis, Cushing syndrome and intracranial hypertension have been reported in children receiving topical corticosteroids. The manifestations of adrenal suppression in children include linear growth delay, delayed weight gain, low levels of plasma cortisol and absence of response to ACTH stimulation. Intracranial hypertension manifestations include lumped fontanelles, headaches and bilateral papillaryem. The administration of topical corticosteroids to children should be limited to the less compatible amount with an effective therapeutic regime. Chronic corticosteroid therapy may interfere with the growth and development of children. WARNING REACTIONS The following local adverse reactions are reported infrequently with topical corticosteroids, but may occur more often with the use of occlusive deposits. These reactions are listed in an approximate order of decreased occurrence: QuemaduraIrritationDrynessFolliculitisHypertrichosisAcneiform EruptionsHypopigmentation Dermatitis by perioralContact allergic Dermatitis Skin macerationInfection by secondarySkin AtrophyStriaeMiliaria OVERDOSAGEOptically applied corticosteroids can be absorbed in sufficient quantities to produce systemic effects (see ). DOSAGE AND ADMINISTRATIONFluocinonide Cream USP, 0.05%, Fluocinonide Cream USP, 0.05% (Emulsified Base), Fluocinonide Gel USP, 0.05% and Fluocinonide Ointment USP, 0.05% generally apply to the affected area as a thin film of two to four times a day depending on the severity of the condition. Occlusive deposits can be used for the management of psoriasis or recalcitrant conditions. If an infection occurs, the use of occlusive deposits should be suspended and appropriate antimicrobial therapy established. HOW SUPPLIEDFluocinonide USP cream, 0.05% is supplied in 15 g (NDC 51672-1253-1), 30 g (NDC 51672-1253-2), 60 g (NDC 51672-1253-3) and 120 g (NDC 51672-1253-4) tubes. Store at 20°-25°C (68°-77°F) [see room temperature controlled by USP]. Fluocinonide Cream USP, 0.05% (Emulsified Base) is supplied in 15 g (NDC 51672-1254-1), 30 g (NDC 51672-1254-2) and 60 g (NDC 51672-1254-3) tubes. Store at room temperature controlled. Avoid excessive heat, above 40°C (104°F). Fluocinonide Gel USP, 0.05% is supplied in 15 g (NDC 51672-1279-1), 30 g (NDC 51672-1279-2) and 60 g (NDC 51672-1279-3) tubes. Store at 20°- 25°C (68°- 77°F) [see room temperature controlled by USP]. Fluocinonide ointment Utensils USP, 0.05% is supplied in 15 g (NDC 51672-1264-1), 30 g (NDC 51672-1264-2) and 60 g (NDC 51672-1264-3) tubes. Store at 20°-25°C (68°-77°F). Avoid temperature above 30°C (86°F). Mfd. de: Taro Pharmaceuticals Inc., Brampton, Ontario, Canada L6T 1C1Dist. de: Taro Pharmaceuticals U.S.A., Inc. Hawthorne, NY 10532Reviewed: November, 2017PK-4964-577PRINCIPAL DISPLAY PANEL - 30 g Tube Carton (Cream) NDC 51672-1253-2 FluocinonideCreate USP, 0.05% Just for extra use. Not for the OPHTHALMIC UTILIA. Keep this and all medicines out of reach of children. 30 g Rx only TARO PRINCIPAL DISPLAY PANEL - 15 g Tube Carton NDC 51672-1254-1 FluocinonideCreate USP, 0.05% (Emulsified Base) Just for extra use. Not for the OPHTHALMIC UTILIA. Keep this and all medicines out of reach of children. 15 g Rx only TARO PRINCIPAL DISPLAY PANEL - 15 g Tube Carton NDC 51672-1279-1 FluocinonideGel USP, 0.05% Just for extra use. Not for the OPHTHALMIC UTILIA. Keep this and all medicines out of reach of children. 15 g Rx only TARO PRINCIPAL DISPLAY PANEL - 15 g Tube Carton NDC 51672-1264-1 FluocinonidaPes de ungüento, 0.05% Just for extra use. Not for the OPHTHALMIC UTILIA. Keep this and all medicines out of reach of children. 15 g Rx only TARO FLUOCINONIDE Fluocinonide cream Product information Product type DRUGItem code (Source)NDC:51672-1253 Ruta de AdministraciónTOPICAL Active Ingredient/Active Mode Name of IngredientBases of strength Fluocinonide (UNII: 2W4A77YPAN) (Fluocinonida - UNII:2W4A77YPAN) Fluocinonida0.5 mg at 1 g Inactive ingredients Name of IngredientForz citric acid monohydrate (UNII: 2968PHW8QP) glycerin (UNII: PDC6A3C0OX) 1.2.6-hexanetriol (UNII: W45XXM0XWE) polyethylene glycol 3350 (UNII: G2M7P15E5P) polyethylene glycol 8000 (UNII: Q662QK8M3B) propilen glycol (UNII: 6DC9Q167V3) (UNII: 2KR89I4H1Y) Packing #Item CodePackage DescriptionMarketing Start dateMarketing End date 1NDC:51672-1253-11 at 1 CARTON06/26/1984 115 g in 1 TUBE; Type 0: Not a combination product 2NDC:51672-1253-21 at 1 CARTON06/26/1984 230 g in 1 TUBE; Type 0: Not a combination product 3NDC:51672-1253-31 at 1 CARTON06/26/1984 360 g in 1 TUBE; Type 0: Not a combination product 4NDC:51672-1253-41 in 1 CARTON06/26/1984 4120 g in 1 TUBE; Type 0: Not a combination product Marketing information Category Marketing Application Number or Monograph Citation End date NDANDA01911706/26/1984 Product information Product type DRUGItem code (Source)NDC:51672-1253 Ruta de AdministraciónTOPICAL Active Ingredient/Active Mode Name of IngredientBases of strength Fluocinonide (UNII: 2W4A77YPAN) (Fluocinonida - UNII:2W4A77YPAN) Fluocinonida0.5 mg at 1 g Inactive ingredients Name of IngredientForz citric acid monohydrate (UNII: 2968PHW8QP) glycerin (UNII: PDC6A3C0OX) 1.2.6-hexanetriol (UNII: W45XXM0XWE) polyethylene glycol 3350 (UNII: G2M7P15E5P) polyethylene glycol 8000 (UNII: Q662QK8M3B) propilen glycol (UNII: 6DC9Q167V3) (UNII: 2KR89I4H1Y) Packing #Item CodePackage DescriptionMarketing Start dateMarketing End date 1NDC:51672-1253-11 at 1 CARTON06/26/1984 115 g in 1 TUBE; Type 0: Not a combination product 2NDC:51672-1253-21 at 1 CARTON06/26/1984 230 g in 1 TUBE; Type 0: Not a combination product 3NDC:51672-1253-31 at 1 CARTON06/26/1984 360 g in 1 TUBE; Type 0: Not a combination product 4NDC:51672-1253-41 in 1 CARTON06/26/1984 4120 g in 1 TUBE; Type 0: Not a combination product Marketing information Category Marketing Application Number or Monograph Citation End date NDANDA01911706/26/1984 FLUOCINONIDE Fluocinonide cream Product information Product type DRUGItem code (Source)NDC:51672-1254 Ruta de AdministraciónTOPICAL Active Ingredient/Active Mode Name of IngredientBases of strength Fluocinonide (UNII: 2W4A77YPAN) (Fluocinonida - UNII:2W4A77YPAN) Fluocinonida0.5 mg at 1 g Inactive ingredients Name of IngredientForz cetyl alcohol (UNII: 936JST6JCN) citric acid anhydrous (UNII: XF417D3PSL) mineral oil (UNII: T5L8T28FGP) 60 (UNII: CAL22UVI4M) propilen glycol (UNII: 6DC9Q167V3) water (UNII: 059QF0KO0R) Sorbian monostea (UNII: NVZ4I0H58X) (UNII: 2KR89I4H1Y) petrolatum (UNII: 4T6H12BN9U) Packing #Item CodePackage DescriptionMarketing Start dateMarketing End date 1NDC:51672-1254-11 at 1 CARTON01/1989 115 g in 1 TUBE; Type 0: Not a combination product 2NDC:51672-1254-21 at 1 CARTON01/1989 230 g in 1 TUBE; Type 0: Not a combination product 3NDC:51672-1254-31 at 1 CARTON01/1989 360 g in 1 TUBE; Type 0: Not a combination product Marketing information Category Marketing Application Number or Monograph Citation End date ANDAANDA07249401/1989 Product information Product type DRUGItem code (Source)NDC:51672-1254 Ruta de AdministraciónTOPICAL Active Ingredient/Active Mode Name of IngredientBases of strength Fluocinonide (UNII: 2W4A77YPAN) (Fluocinonida - UNII:2W4A77YPAN) Fluocinonida0.5 mg at 1 g Inactive ingredients Name of IngredientForz cetyl alcohol (UNII: 936JST6JCN) citric acid anhydrous (UNII: XF417D3PSL) mineral oil (UNII: T5L8T28FGP) 60 (UNII: CAL22UVI4M) propilen glycol (UNII: 6DC9Q167V3) water (UNII: 059QF0KO0R) Sorbian monostea (UNII: NVZ4I0H58X) (UNII: 2KR89I4H1Y) petrolatum (UNII: 4T6H12BN9U) Packing #Item CodePackage DescriptionMarketing Start dateMarketing End date 1NDC:51672-1254-11 at 1 CARTON01/1989 115 g in 1 TUBE; Type 0: Not a combination product 2NDC:51672-1254-21 at 1 CARTON01/1989 230 g in 1 TUBE; Type 0: Not a combination product 3NDC:51672-1254-31 at 1 CARTON01/1989 360 g in 1 TUBE; Type 0: Not a combination product Marketing information Category Marketing Application Number or Monograph Citation End date ANDAANDA07249401/1989 FLUOCINONIDE Fluocinonide gel Product information Product type DRUGItem Code (Source)NDC:51672-1279 Ruta de AdministraciónTOPICAL Active Ingredient/Active Mode Name of IngredientBases of strength Fluocinonide (UNII: 2W4A77YPAN) (Fluocinonida - UNII:2W4A77YPAN) Fluocinonida0.5 mg at 1 g Inactive ingredients Name of IngredientForz carbomer type c (pentaerythritol crosslinked) (UNII: 4Q93RCW27E) edetate disodium (UNII: 7FLD91C86K) propyl (UNII: 8D4SNN7V92) propilen glycol (UNII: 6DC9Q167V3) sodium hydroxide (UNII: 55X04QC32I) water (UNII: 059QF0KO0R) Packing #Item CodePackage DescriptionMarketing Start dateMarketing End date 1NDC:51672-1279-11 at 1 CARTON07/29/1997 115 g in 1 TUBE; Type 0: Not a combination product 2NDC:51672-1279-21 in 1 CARTON07/29/1997 230 g in 1 TUBE; Type 0: Not a combination product 3NDC:51672-1279-31 in 1 CARTON07/29/1997 360 g in 1 TUBE; Type 0: Not a combination product Marketing information Category Marketing Application Number or Monograph Citation End date ANDAANDA07493507/29/1997 Product information Product type DRUGItem Code (Source)NDC:51672-1279 Ruta de AdministraciónTOPICAL Active Ingredient/Active Mode Name of IngredientBases of strength Fluocinonide (UNII: 2W4A77YPAN) (Fluocinonida - UNII:2W4A77YPAN) Fluocinonida0.5 mg at 1 g Inactive ingredients Name of IngredientForz carbomer type c (pentaerythritol crosslinked) (UNII: 4Q93RCW27E) edetate disodium (UNII: 7FLD91C86K) propyl (UNII: 8D4SNN7V92) propilen glycol (UNII: 6DC9Q167V3) sodium hydroxide (UNII: 55X04QC32I) water (UNII: 059QF0KO0R) Packing #Item CodePackage DescriptionMarketing Start dateMarketing End date 1NDC:51672-1279-11 at 1 CARTON07/29/1997 115 g in 1 TUBE; Type 0: Not a combination product 2NDC:51672-1279-21 in 1 CARTON07/29/1997 230 g in 1 TUBE; Type 0: Not a combination product 3NDC:51672-1279-31 in 1 CARTON07/29/1997 360 g in 1 TUBE; Type 0: Not a combination product Marketing information Category Marketing Application Number or Monograph Citation End date ANDAANDA07493507/29/1997 FLUOCINONIDE ointment of fluocinonide Product information Product type DRUGItem Code (Source)NDC:51672-1264 Ruta de AdministraciónTOPICAL Active Ingredient/Active Mode Name of IngredientBases of strength Fluocinonide (UNII: 2W4A77YPAN) (Fluocinonida - UNII:2W4A77YPAN) Fluocinonida0.5 mg at 1 g Inactive ingredients Name of IngredientForz glicéril monostearate (UNII: 230OU9XE4) Propylene carbonate (UNII: 8D08K3S51E) propilen glycol (UNII: 6DC9Q167V3) petrolatum (UNII: 4T6H12BN9U) White wax (UNII: 7G1J5DA97F) Packing #Item CodePackage DescriptionMarketing Start dateMarketing End date 1NDC:51672-1264-11 at 1 CARTON06/30/1999 115 g in 1 TUBE; Type 0: Not a combination product 2NDC:51672-1264-21 at 1 CARTON06/30/1999 230 g in 1 TUBE; Type 0: Not a combination product 3NDC:51672-1264-31 at 1 CARTON06/30/1999 360 g in 1 TUBE; Type 0: Not a combination product Marketing information Category Marketing Application Number or Monograph Citation End date ANDAANDA07500806/30/1999 Product information Product type DRUGItem Code (Source)NDC:51672-1264 Ruta de AdministraciónTOPICAL Active Ingredient/Active Mode Name of IngredientBases of strength Fluocinonide (UNII: 2W4A77YPAN) (Fluocinonida - UNII:2W4A77YPAN) Fluocinonida0.5 mg at 1 g Inactive ingredients Name of IngredientForz glicéril monostearate (UNII: 230OU9XE4) Propylene carbonate (UNII: 8D08K3S51E) propilen glycol (UNII: 6DC9Q167V3) petrolatum (UNII: 4T6H12BN9U) White wax (UNII: 7G1J5DA97F) Packing #Item CodePackage DescriptionMarketing Start dateMarketing End date 1NDC:51672-1264-11 at 1 CARTON06/30/1999 115 g in 1 TUBE; Type 0: Not a combination product 2NDC:51672-1264-21 at 1 CARTON06/30/1999 230 g in 1 TUBE; Type 0: Not a combination product 3NDC:51672-1264-31 at 1 CARTON06/30/1999 360 g in 1 TUBE; Type 0: Not a combination product Marketing information Category Marketing Application Number or Monograph Citation End date ANDAANDA07500806/30/1999 Tags - Taro Pharmaceuticals U.S.A., Inc. (145186370) Establishment NameAddressID/FEIBusiness Operations Taro Pharmaceuticals Inc.206263295MANUFACTURE(51672-1253, 51672-1254, 51672-1279, 51672-1264)

Fluocinonide Cream USP, 0.05% Fluocinonide Cream USP, 0.05% (Emulsified Base) Fluocinonide Gel USP, 0.05% Fluocinonide Ointment USP, 0.05%

Fluocinonide Cream USP, 0.05% Fluocinonide Cream USP, 0.05% (Emulsified Base) Fluocinonide Gel USP, 0.05% Fluocinonide Ointment USP, 0.05%
What is Fluocinonide? - GoodRx

Fluocinonide Cream 0.05% 15 Gram — Mountainside Medical Equipment

Fluocinonide Cream USP, 0.05% Fluocinonide Cream USP, 0.05% (Emulsified Base) Fluocinonide Gel USP, 0.05% Fluocinonide Ointment USP, 0.05%
What is Fluocinonide-E? - GoodRx
What is Fluocinonide-E? - GoodRx

Fluocinonide Cream 0.05% 15 Gram — Mountainside Medical Equipment

Doc Lanes

Fluocinonide Ointment - Setting a new standard for the future of pharma

Fluocinonide Cream 0.05% 30gm Tube 30gm/Tb - Henry Schein Special Markets

Fluocinonide Gel, USP 0.05% - Teligent
What is Fluocinonide? - GoodRx

Fluocinonide Topical Solution USP 0.05% - Teligent
FLUOCINONIDE 0.05% CREAM [TARO] - First Veterinary Supply

Fluocinonide Topical Solution - Setting a new standard for the future of pharma

FLUOCINONIDE 0.05% (LIDEX E) CRM 60GM

FLUOCINONIDE CREAM USP, 0.05% (Emulsified Base) FLUOCINONIDE CREAM USP, 0.05%FLUOCINONIDE OINTMENT USP, 0.05% 026202630264 FOR TOPICAL/EXTERNAL USE ONLYNOT FOR OPHTHALMIC USERx only

Fluocinonide Cream

Fluocinonide Solution - FDA prescribing information, side effects and uses

FLUOCINONIDE TOPICAL SOLUTION, USP 0.05% Rx only

FLUOCINONIDE TOPICAL: Lidex, Various - Top 300 Pharmacy Drug Cards

Taro 51672127901 - McKesson Medical-Surgical

Fluocinonide Ointment USP, 0.05% - Teligent

Fluocinonide Topical - Prescriptiongiant

Fluocinonide Ointment USP, 0.05% - Teligent
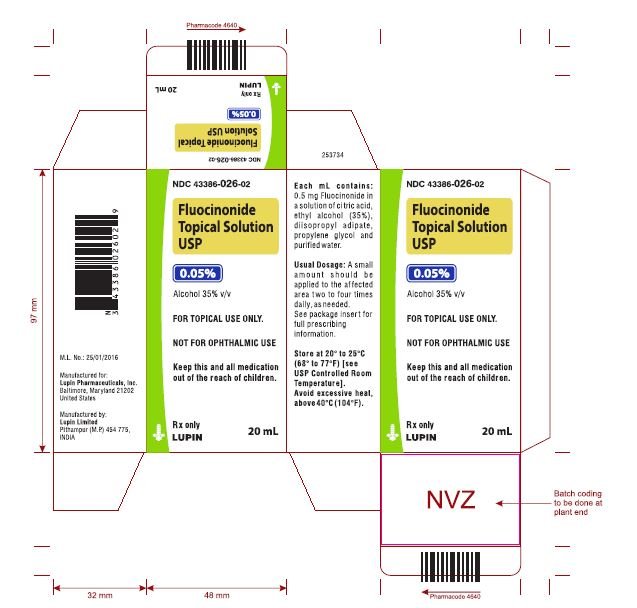
Fluocinonide Solution - FDA prescribing information, side effects and uses

Buy Rx: Lidex 500 mcg / g 5 g Ointment Online | Southstar Drug

FLUOCINONIDE TOPICAL SOLUTION, USP 0.05% Rx only

Fluocinonide Gel - FDA prescribing information, side effects and uses

Fluocinonide Cream 0.05% 30gm By Taro Pharmaceuticals

Fluocinonide Ointment 0.05% 30g

Fluocinonide 0.05%, Cream, 60gm Tube | McGuff Medical Products
Fluocinonide Topical | Medline Industries, Inc.

Fluocinonide Ointment - FDA prescribing information, side effects and uses

Fluocinonide Ointment 0.05% (RX), 60 gm Alvogen, Inc. Ingredients and Reviews

FLUOCINONIDE TOPICAL SOLUTION USP, 0.05%

Fluocinonide Ointment Tube 0.05% 15g/Tb - Henry Schein Special Markets
What is Fluocinonide? - GoodRx

Fluocinonide Ointment - FDA prescribing information, side effects and uses
Posting Komentar untuk "fluocinonide .05%"